Summary
• Vaccines for Covid-19 are already being administered in some countries, and will be widely available in many countries by the first half of 2021.
• Given the limited supply, swift decision-making is needed to take an action on procurement and distribution of the vaccine in Pakistan.
• Policymakers must consider establishing a parliamentary level committee that considers all aspects pertaining to vaccine procurement and distribution, in addition to tackling mis-information
and ensuring an effective tech-based monitoring mechanism for all aspects of vaccination. The special committee should focus on coordination among the provinces and centre.
• To fulfill the needs of a growing Pakistani population, Pakistan needs a policy around the issue of vaccine and biotechnology and appropriate incentive mechanisms to encourage local production over the long term.
Covid-19 Vaccine Landscape
Globally, the research for a vaccine for the novel coronavirus (or Covid-19) started immediately after the virus surfaced in Wuhan, China. Normally, it takes considerable time and resources to produce and fully develop a vaccine for treatment. However, in the case of Covid-19, researchers worldwide have worked around the clock to develop a vaccine in record time. Vaccines are already being administered in some countries, and will be widely available in many others by the first half of 2021.
Vaccine development has different phases before it is approved for mass manufacturing. At the moment, there are more than 150 vaccines in the pre-clinical phase, which means they are being explored in lab experiments. Twenty (20) different vaccines are in Phase I, where they are being tested on healthy young individuals for safety tests. There are eleven (11) vaccines in Phase II, which are being tested on a broad-er and more diverse group of people. And there are only eleven (11) vaccines in Phase III. These will be available for general use once they are approved and licensed.
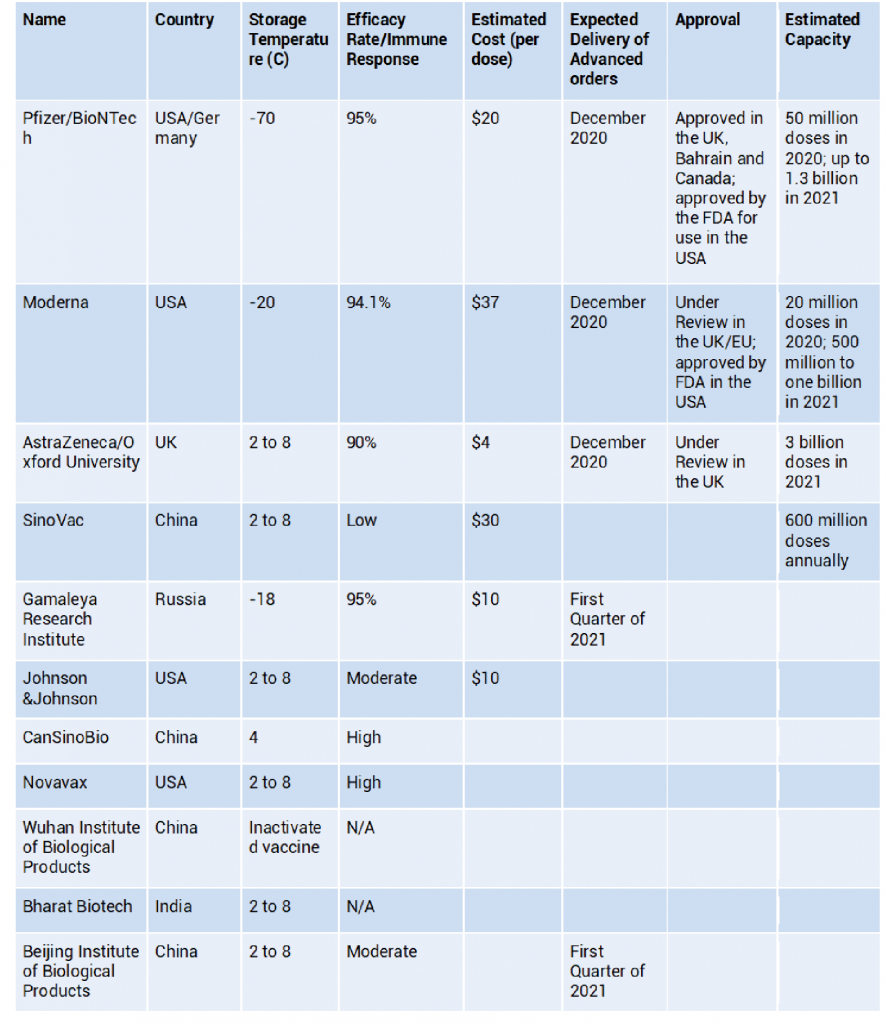
These eleven vaccines in Phase III are seen as the solution to deal with the spread of the SARS-CoV-2. Below are details on each of these eleven leading candidates:
Global Developments
The United Kingdom was the first country to approve the Pfizer/BioNTech vaccine for general use [i]. Bahrain has also approved Pfizer/BioNTech vaccine, which will be used for high-risk groups [ii]. U.S. Food and Drug Administration (FDA) has also authorized the Pfizer/BioNTech vaccine for use in the USA. Pfizer can only produce a limited quantity of the vaccine over the next year — around 1.3 billion doses. About 1.1 billion doses, more than 80% of the supply, are booked through advanced purchase agreement by the United States, the United Kingdom, the EU, Canada and Japan [iii]. FDA has also recently given Emergency Use Authorization (EUA) to Moderna, making it the second to receive such authorization in the USA.
China has five vaccine candidates that are in phase three trials globally. CanSinoBio’s vaccine is approved by the Chinese Military for 1 year as a “specially needed drug”. SinoVac has also received emergency approval by the Chinese government for limited use [iv]. The vaccine by Wuhan Institute has received emergency approval in the UAE for frontline health workers [v].
Russia developed the first registered vaccine for Covid-19. However, it was seen to be pre-mature as before it went beyond the first phase, the vaccine was approved for use by the Russian government. Nonetheless, the vaccine right now is going through the third phase with trials happening in Latin American and Middle East. The vaccine is said to be available for supply starting next month [vi], and more than 50 countries have requested to purchase the vaccine from Russia [vii].
Indian government is planning to release the vaccine by January next year; the Covishield vaccine made by Serum Institute of India, which has partnered with British pharmaceutical company AstraZeneca; and Covaxin, being developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) have already applied for authorisation. The development of vaccine locally signals that the Indian government does not want to rely only on other countries or manufacturers, particularly given its considerable demand due to its enormous population size [viii]. Bharat Biotech is the biggest vaccine manufacturer in the world by volume, and they plan to make their vaccine available for a cost “less than a water bottle”.
WHO, CEPI, and Gavi: The Vaccine Alliance, came up with a joint initiative called COVAX. The purpose of this coalition is to “provide countries worldwide equitable access to safe and effective vaccines, once they are licensed and approved [ix].” The alliance works with both governments and manufacturers to make sure that vaccines are available to both high-income and low-income countries [x]. The US has not contributed to the initiative, but has signed bilateral deals with various vaccine makers, including non-US pharmaceutical companies, which is an indication of Trump’s America First policy, but this might change under Joe Biden.
National Developments
Pakistan is currently experiencing the second wave of the Covid-19 pandemic, which makes it even more critical to procure a vaccine as soon as possible. The federal government has allocated $150 million to procure a vaccine. The plan is to cover healthcare workers and citizens aged 65 and above (a total of about 10 million) in Phase I. Special Assistant to the Prime Minister on Health, Dr. Faisal Sultan, said that they will consider factors such as the type of vaccine, storage requirements, cost, production capacity, procurement access, the efficacy of the vaccine, and its side effects while deciding which vaccine to procure [xi].
Under the COVAX initiative, Pakistan, like other countries, is committed vaccination for 20% of the population [xii]. Pakistan now meets all the conditions to benefit from COVAX facility, which will lead to access to donor-funded doses [xiii]. Through COVAX, Pakistan is expected to receive the vaccine for 45 million Pakistanis for free, and it also has a provision to purchase more later [xiv].
Pakistan will begin the first vaccine drive by April of 2021 and it is planned to be provided free of charge [xv]. A distribution strategy, is also under development by the government. The government has also paved the way for distribution and coordination of the vaccine by providing tax exemption for 61 items used in Covid-19 vaccine preparation [xvi]. Chinese vaccine manufactured by CanSinoBio is currently going through phase 3 trial in Pakistan, which is being led by the National Institute of Health along with AJM Pharma.
Implications for Pakistan
Globally, the biggest challenge will be avoiding “vaccine nationalism”, where countries stock up huge piles of vaccine supply, and vaccine distribution depends on a country’s purchasing power. In the past, this has happened when a vaccine for H1N1 became available; as wealthier countries bought up the supplies, it left poorer nations at the back of the line. An assessment shows that the US, EU, UK and Canada have pur-chased or ordered enough doses that will cover 8, 4, 7, and 11 doses per person, respectively [xvii].
The government has allocated around $150 million for procurement of vaccine. This is a good initiative but given a lot of countries have directed their funds towards pre-orders, the Pakistani government needs to follow suit as well. Pakistan does not have the capability and capacity to produce vaccines itself; this is due to a lack of policy and vision, and an area that has not received much focus [xviii].
Through the COVAX initiative, Pakistan is expected to receive vaccines for 20% of the population but relying on only that as a strategy will not be enough as to achieve “herd immunity”, it requires between 55% to 82% of the population (approx. 160 million people) to be immune either through vaccination or prior infection. The availability of vaccine under the COVAX initiative is also subject to funding availability at their end.
From the above mentioned candidates, procuring a vaccine from either Pfizer or Moderna would be a challenge for Pakistan, as more than 80% of their supply is already booked for by the developed countries, and the challenge, for low to middle-income countries like Pakistan is that they will not be able to get it before the end of next year. At the same time, their vaccine is considerably expensive compared with other candidate vaccines. Another challenge with mRNA-based vaccines is cold chain storage, which will be costly for Pakistan to establish since the current system cannot cater to the required storage temperature of -70°C as is the case for Pfizer’s vaccine or -20 for Moderna [xix].
Even though the supply is short, and Pakistan will have to make do with its limited resources, a major advantage Pakistan has is its close partnership and cordial relations with China. To further its “vaccine diplomacy”, China has decided to prioritize providing the vaccine to countries that benefit from its Belt and Road Initiative and other countries; which includes Indonesia, Bangladesh, Pakistan and Morocco. Beijing recently joined the COVAX initiative as well, in hopes that it will be able to clear its name globally which came under fire as the virus originated from China [xx]. Pakistan can take advantage of its diplomatic relationship with Beijing to procure vaccine.
Procurement of the vaccine is an important factor; equally important is public acceptance and trust towards the same. For instance, even in a developed country like the United States, only 56% of people are willing to receive a Covid-19 vaccine [xxi]. In Pakistan also, this is a big challenge since in the past with Polio and other vaccines, the public has displayed mistrust and is a reason why Pakistan has not been able to eliminate polio. There are already conspiracy theories and false narrative around the vaccine. In this regard, consistent public messaging is very important. Experts have suggested that there is a need for political unity and national coordination and consensus when it comes to dealing with the Covid-19 pandemic, and a parliamentary committee that has a mandate to ensure unity and inter-provincial coordination on a Covid-19 vaccine can be a step in the right direction.
More importantly, the government must continue mitigating the transmission of the virus, which can be only done through the protection of vulnerable groups and ensuring that citizens adhere to public health measures. The vaccine must be seen as a mid to long-term solution, and the policymakers must steer clear of creating a narrative that the virus is defeated before the vaccine even arrives.
Covid-19 pandemic had a major impact on international travel and mobility, which has not just impacted the tourism sector but also businesses. It has become mandatory for travelers to get a Covid-19 RT-PCR test before international travel. As the vaccine becomes available in more and more countries, it will eventually become a requirement for travelers to carry a “vaccine passport” with them, which will ensure that they have been vaccinated and hence they can travel freely. As we look towards post-Covid-19 economic recovery, ensuring vaccination will become important.
Recommendations
1
An important first step is to establish a national level parliamentary committee that will deal with the selection, procurement, distribution, and storage of the vaccine and operate through sub-committees on specific areas. The special committee should focus on coordination among the provinces and centre while working in coordination with the NCOC and SAPM Health and must in-clude experts from the field of virology.
2
Through consultations with scientists, doctors, and experts, the government should make use of the allocated money to place advance orders of the vaccine from the above mentioned candidates. Delay on this will further push Pakistan down on the list of the producers as more and more countries are placing advance orders.
3
Pakistan lacks a policy around the issue of vaccine and biotechnology. Policymakers should consult with experts in the field to create a policy and create incentives through policy changes for pharmaceutical industries to pursue local vaccine production, which will be even more integral in the future to fulfil the needs of a growing Pakistani population.
4
The government should very carefully focus on trust-building mechanisms which can increase public trust and acceptance towards the forthcoming vaccine. Such a behaviour-change strategy is necessary, given the country’s experience with vaccination and current false narratives regarding Covid-19 itself. Awareness campaigns which focus on debunking conspiracies and also build on the experience with the polio campaign must be broadcasted across multiple channels to enhance acceptance. Coherence in public messaging by the government is extremely essential.
This material has been developed by Tabadlab in partnership with DRI. It has been funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
End Notes
[i] Al Jazeera. UK approves Pfizer-BioNTech vaccine for use in world first. 2 December 2020.
https://www.aljazeera.com/news/2020/12/2/uk-approves-pfizer-biontech-vaccine-for-use-first-in-the-world.
[ii] Al Jazeera. Bahrain becomes second country to approve Pfizer COVID-19 vaccine. 4 December 2020.
https://www.aljazeera.com/news/2020/12/4/bahrain-becomes-second-country-to-approve-pfizer-covid-19-vaccine.
[iii] Basharat, Rahul. Pakistan to get around 20m doses of COVID-19 vaccine. 18 November 2020.
https://nation.com.pk/18-Nov-2020/pakistan-to-get-around-20m-doses-of-covid-19-vaccine.
[iv] BBC. Covid-19: Chinese vaccine ‘successful in mid-stage trials. 18 November 2020.
https://www.bbc.com/news/world-asia-china-54982910.
[v] Chandna, Himani. Sputnik V Covid vaccine likely to be available for supply by next month, says Russia’s RDIF. 1 December 2020.
https://theprint.in/health/sputnik-v-covid-vaccine-likely-to-be-available-for-supply-by-next-month-says-russias-rdif/555174/.
[vi] Das, Krishna N. Exclusive: India-made COVID-19 vaccine could be launched as early as February – government scientist. 5 November 2020.
https://www.reuters.com/article/us-health-coronavirus-india-vaccine-excl/exclusive-india-made-covid-19-vaccine-could-be-launched-as-early-as-february-government-scientist-idUSKBN27L0XA.
[vii] DAWN. Pakistan hopes to procure Covid-19 vaccine by first quarter of 2021: Dr Faisal Sultan. 01 December 2020.
https://www.dawn.com/news/1593437/pakistan-hopes-to-procure-covid-19-vaccine-by-first-quarter-of-2021-dr-faisalsultan.
[viii] DAWN. Tax exemption for 61 items for vaccine approved. 18 November 2020.
https://www.dawn.com/news/1590934/tax-exemption-for-61-items-for-vaccine-approved
[ix] DAWN. Is Pfizer’s vaccine the answer to Pakistan’s Covid-19 problem? 13 November 2020.
https://www.dawn.com/news/1589993/is-pfizers-vaccine-the-answer-to-pakistans-covid-19-problem.
[x] Debinski & Winkleman. The Graphic Truth: Who’s buying up the COVID vaccines? 4 December 2020.
https://www.gzeromedia.com/the-graphic-truth-whos-buying-up-the-covid-vaccines.
[xi] GAVI. The COVID-19 vaccine race. 2 December 2020.
https://www.gavi.org/vaccineswork/covid-19-vaccine-race.
[xii] Hilotin, Jay. How 172 countries raised $18 billion for COVID-19 vaccine, and the role of Indian biotech firms. 24 August 2020.
https://gulfnews.com/world/how-172-countries-raised-18-billion-for-covid-19-vaccine-and-the-role-of-indian-biotech-firms-1.1600608348925.
[xiii] Howard, Jacqueline. The percentage of Americans who say they would get a Covid-19 vaccine is falling, CNN poll finds. 6 October 2020.
https://edition.cnn.com/2020/10/05/health/covid-19-vaccine-willingness-cnn-poll-wellness/index.html
[xiv] Khalid, Hamnah. Pakistan now fulfils all criteria to order COVID-19 vaccines. 4 December 2020.
https://www.techjuice.pk/pakistan-now-fulfils-all-criteria-to-order-covid-19-vaccines/.
[xv] Khan, Yusra Habib et al. “Threat of COVID-19 Vaccine Hesitancy in Pakistan: The Need for Measures to Neutralize Misleading Narratives.” The American journal of tropical medicine and hygiene vol. 103,2 (2020): 603-604.
[xvi] Latif, Aamir. Pakistan to start COVID-19 vaccination drive in April. 2 December 2020.
https://www.aa.com.tr/en/asia-pacific/pakistan-to-start-covid-19-vaccination-drive-in-april/2063354.
[xvii] Moderna. “Moderna Announces Primary Efficacy Analysis in Phase 3 COVE Study for Its COVID-19 Vaccine Candidate and Filing Today with U.S. FDA for Emergency Use Authorization.” 30 November 2020.
https://investors.modernatx.com/node/10421/pdf.
[xviii] NPR. Why Poorer Countries Aren’t Likely To Get The Pfizer Vaccine Any Time Soon. 11 November 2020.
https://www.npr.org/sections/goatsandsoda/2020/11/11/933942711/why-poorer-countries-arent-likely-to-get-the-pfizer-vaccineany-time-soon.
[xix] PT. Janssen begins second Phase III Covid-19 vaccine trial . 16 November 2020.
https://www.pharmaceutical-technology.com/news/janssen-ensemble-2-trial/.
[xx] Reuters. UAE announces emergency approval for use of COVID-19 vaccine. 14 September 2020.
https://www.reuters.com/article/us-health-coronavirus-emirates-vaccine/uae-announces-emergency-approval-for-use-of-covid-19-vaccine-idUSKBN2652OM.
[xxi] The Moscow Times. Russia’s Sputnik V Coronavirus Vaccine ‘Reasonably Effective’ – British Experts. 30 November 2020.
https://www.themoscowtimes.com/2020/11/30/russias-sputnik-v-coronavirus-vaccine-reasonably-effective-british-experts-a72182.
Muhammad Asad Rafi is a Research Analyst for Global Affairs and International Security at Tabadlab. He holds a M.A in International Security from Paris School of International Affairs at Sciences Po. His research interests lie in the fields of regional security, foreign policy and global affairs.






